Volume 10 issue 7
What’s Happening at Missouri S&T:
(formerly UMR)
Short Course Dates
We will be offering "Basic Composition of Coatings" March 24-28,2014 (Spring 2014). The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations.
We will be offering "Introduction to Paint Formulation" May 19-23, 2014 (Spring 2014). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
We will be offering "Introduction to Coatings Composition and Specifications" July 21-23, 2014 (Summer 2014), course designed for the new coatings person in areas such as sales, marketing or production. The course was initiated by a number of raw material companies and distributors requesting a course with this format. This course is not as heavily technical as is our “Basic Composition of Coatings" and “Introduction to Paint Formulation" courses. The ?Introduction to Coatings Composition and Specifications" course is a two and a half day course which will discuss the types of coatings, the basic composition of coatings and the tests and specifications used by the industry. This course will allow the participant to gain the fundamentals needed to work in this industry and to communicate more clearly.
For more information see our web site at http://coatings.mst.edu and to register contact Catherine Hancock at cemv26@mst.edu or coatings@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
Online Short Course
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
Employment Tab
We have started an employment section for our students and companies. We have a full time job section, an intern / co-op section and a graduating and alumni students section . Please explore our section on employment on our web site. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: svgwcc@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
Technical Insights on coatings Science
Protecting Paint from Microorganisms
Catherine E. Hancock, Graduate Student
Missouri S&T Coatings Institute
Microorganisms are ever-present in the environment. Many waterborne coatings have the necessary environment for the reproduction of bacteria or fungi resulting in the need for both in-can preservatives and dry-film preservatives. Generically, the name of the preservative explains its purpose. An in-can preservative protects the paint from any deterioration before application, extending shelf life, while in transit and the dry-film preservative protects the paint after it has been applied and dried ensuring the aesthetic look and an extended performance of the coating.
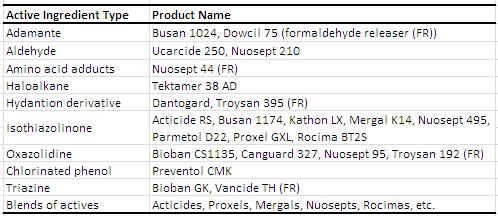
In-can preservatives are used in waterborne coatings to prevent spoilage due to microbial containments, most commonly bacteria, but organisms like yeast and fungi can cause spoiling as well. The most common bacteria that cause deterioration are Aeromonas sp., Escherchia sp., Enteroacter sp., Pseudomonas sp, and rarely, Bacillus sp., and Desulfovibrio sp.1 Contamination can arise from various processes including the water source, infected raw materials, defective paint cans, and poor hygiene. The use of an in-can preservative will aid to reduce the effect of microbial contamination and prevent further microbial proliferation. Generally, broad-spectrum preservatives are used. Of course, testing must be done to optimize cost and efficiency and to analyze its effect on the coating physical properties, such as pH, viscosity, odor, and color change. There are many compounds and functional groups utilized as in-can preservatives. Choosing the right in can preservative is not simple. One biocide (active ingredient) is not interchangeable to other. Many of the active ingredients listed below release formaldehyde as the mechanism of action and the use in green label paint is restricted. Non- formaldehyde releaser active ingredients that are suitable for alkaline pH matrix such as paint are very limited.2 Clear understanding of the paint system is needed to obtain an optimum in can preservative system. The table below is taken from the Coatings Technology Handbook and is a list of common in-can preservatives available on the market.1
Examples of In-Can Preservatives1 (edited for 2013)
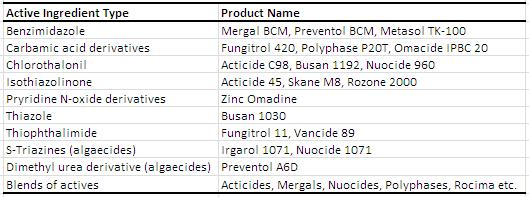
Waterborne and solvent borne coatings are susceptible to fungi and algae growth in a dry film. Growth can occur due to a variety of hosts such as carbon source, temperature, moisture, degradation of nutrient rich additives, porous substrate and pH. When a coating is damaged due to growth, it is ‘defaced’ or has defacement. Not only are the algae and fungal growth unsightly, it can cause property damage such as blistering and cracking. Again, the table below from the Coating Technology Handbook lists several popular dry-film preservatives on the market. Just like in the in-can preservatives case, only a very few active ingredients can be used for green label due to health or environmental concerns.
Examples of Dry-Film Biocides1 (edited for 2013)
Though many of the preservatives suppliers utilized their own internal method to conduct optimization study, in general, ASTM methods can be used as a basic guideline. For in-can preservatives ASTM d5588-97 is commonly followed. For dry-film preservatives outdoor coatings are generally tested by accelerated methods, ASTM D5590-94 and ASTM D5589-97, where coatings are inoculated directly with fungi or algae and the coating susceptibility is measured. (ASTM D3456-86 is used for long term outdoor exposure testing). In the case of interior coatings, a controlled environment chamber is utilized (ASTM D3273-86).
Preservatives work in a variety of manners, but most of them damage cell walls or membranes, or interfere within the cell functions such as energy production, respiratory system, efflux function, etc. If you remember back to basic biology, one of the very simple but very distinguishable difference between an animal cell VS a plant or bacteria cell is the cell wall. Only plant and bacteria have a cell wall, so many preservatives use this advantage; hence the damage is done to the wall or membrane of the microbial, not to the animal cell. The synthesis of the wall can be prohibited, similar to the function of penicillin.3 Quaternary ammonium derivatives are a common biocide that uses this mechanism but is not commonly used in paint. The other method involves the inhibition of protein synthesis and other cell functions by diffusing into the cell and reacting with –SH groups of glutathione, protein, amino acids, disulfides, etc. This is a slower starvation of the cell compared to the interruption of the wall or membrane.
Preventing microbial growth control is necessary in both in-can and dry-film, and must agree with the other components of the system. The typical use of preservatives ranges from 0.05 to 0.3 weight percent for in can preservation and 0.25 to 1% for dry film preservation depending on length of protection needed. All preservatives are highly regulated and have to be registered by EPA (USA) and other regulatory agency of specific countries where these products are sold before can be used for this type of applications. The use concentrations are also limited to what is approved in the label by regulatory body (EPA-USA).
Reference:
1. Tracton, A. Coatings Technology Handbook 3rd Edition. 2006, CRC Press, Chapter 81
2. Paulus, W. Microbiocides for the Protection of Materials: A Handbook. 1993, Chapman & Hall. p 55
3. Todar, K. Online Textbook of Bacteriology. 2008-2012. http://textbookofbacteriology.net/antimicrobial_3.html